Another Update on Drug Price Disclosure Legislation
More tips for staying compliant with an accelerating trend
States Continue Their March Toward Prescription Drug Transparency Regulations.
This paper, our fourth in a series that looks at the rising number of price transparency
laws and their attention-grabbing compliance ramifications for pharma companies, offers tips on preparing for this new reality.
As we enter a new year, pharmaceutical manufacturers enter a more complex environment when it comes to the various State Price Transparency requirements. More states have rolled out new price transparency legislation since our last update in mid-2020. Three states have implemented, or are in the process of implementing, new laws and reporting requirements. This brings the total count to 21 states, up from 18 last year.
The new states to take note of: Minnesota, Maryland, and Utah. Some reporting deadlines surrounding these states have already come and gone. Also of note, as of October 2020, Washington opened its online portal for data submission.
As you will see in the chart below, the three new states have not passed legislation that requires any grueling amount of reporting. Nonetheless, it’s important to know they have joined the growing list, as falling out of compliance with any state puts a manufacturer at risk of getting hit with large civil penalties.
Know Which State Reporting Requirements May Apply to You
As the number of states that have enacted price transparency laws increases so does the task of staying in compliance. Not only has the number of laws increased, but each of these states has its own set of nuances for each specific law that they have rolled out. The price increase you may need to report to one state, you may not need to report to another. It is important to read the fine print regarding each requirement for each state.
DRUG PRICE TRANSPARENCY LAWS CATEGORIZED BY TYPE AND STATE
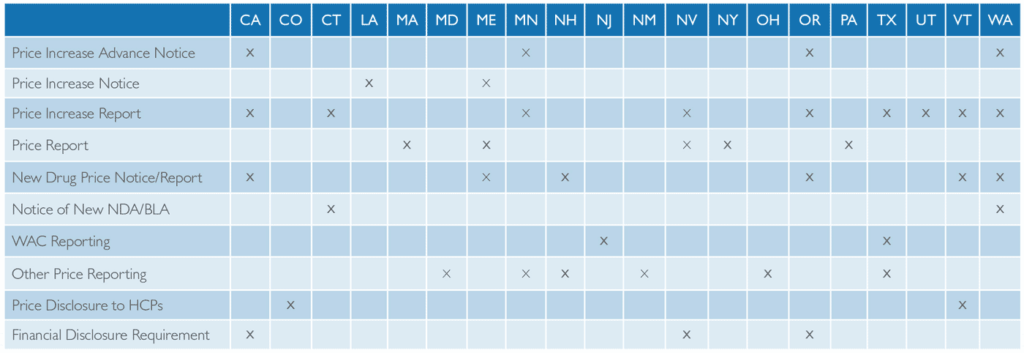
An at-a-glance look at all 21 states that currently have pharmaceutical drug price disclosure laws, and the types of laws enacted. Source: Prescription Analytics, state regulatory monitoring
MARYLAND – IN SUMMARY
Maryland’s legislation has created the Prescription Drug Affordability Board. While there are no specific reporting requirements for manufacturers to abide by on a quarterly or annual basis like other states, the board may request information from manufacturers as it deems necessary. The board has the right to review select prescription drugs and set upper limits for prescription drug products purchased or paid for by the State. Based on this review, the board may request additional information from the manufacturer. Drugs are eligible if they are brand name drugs or biologics whose launch WAC is $30,000 or more per year or have had a WAC increase of $3,000 or more per year; or biosimilars who have a launch WAC that is not at least 15% lower than the reference brand biologic; or a generic drug with a WAC of $100 or more for a 30-day supply and increased by 200% or more from the immediately preceding 12-month period. Given the complexity of those requirements, we can breathe a sigh of relief that the Board is in control and will request information if required.
MINNESOTA – IN SUMMARY
The state of Minnesota has rolled out new requirements that closely mirror that of other states. Aspects of Minnesota’s law includes new drug price reporting, newly acquired drug reporting, and price increase reporting.
Beginning October 1, 2021, manufacturers must submit new drug reports no later than 60 days after launching a new drug that is greater than the tier threshold set by Medicare Part D (currently $670) for a 30-day supply. Manufacturers must also report any newly acquired drugs with a WAC over $100 for a 30-day supply and if there is an increase in the price of greater than 16% for a branded drug or 50% for a generic drug. Price increase reporting is required for drugs with a WAC of $1,000 or more for a 30-day supply and has undergone a WAC increase of 16% or more for branded drugs or 50% or more for generic drugs. At this point in time, Minnesota has not stated how it plans to collect this information from manufacturers.
UTAH – IN SUMMARY
Utah recently rolled out new legislation and required all manufacturers to register with the State by October 30, 2020 at the latest. If you are a manufacturer and have not yet registered, it would be wise to do so. The State is still working out the details of the new legislation, but at this point has a price increase reporting requirement. Utah will require manufacturers to report WAC increases that are greater than 16% over the preceding two calendar years, or 10% over the preceding calendar year. However, the State has not yet revealed how it will collect this data or what template will be used. The Utah Insurance Commissioner has stated that drug manufacturer reports will not be required prior to January 1, 2022 as the this will give the State time to develop a reporting template, and administrative rule, and a standard method of securely transferring and storing information.
WASHINGTON – UPDATE
As of the fall of 2020, the state of Washington has launched an online submission portal for manufacturers to access and submit any required information. At the time of our last writing, Washington had not yet begun to collect information from manufacturers as it had no way of doing so.
Washington has various reporting requirements as follows. Price increase reporting is required if the WAC is more than $100 and increases by either 20% annually or 50% from the prior three calendar years. Price-increase advanced notice is required if a WAC increase triggers a 20% annual or 50% (three year) threshold. Manufacturers must submit the notice and detailed report 60 days in advance. For an ANDA or biosimilar, if not possible to provide an increase 60 days in advance, this information is due no later than effective date of increase. New drug reporting is required for any newly launched drug with a WAC of $10,000 or greater for a 30-day supply. Last, and a fairly uncommon requirement to date, Washington requires manufacturers to submit notice to the State of new FDA approvals for NDAs and BLAs within 60 days of receiving FDA approval.
LOOKING FORWARD
There is currently little on the horizon that would suggest any relief on the state price reporting front, at least in the foreseeable future. Just the opposite is likely as the number of states enacting their own version of pharmaceutical price transparency requirements continues to grow. More state legislatures jumping on the bandwagon with their own version of price disclosure rules, while not significantly different than existing regulations, will still result in additional complexity for pharmaceutical manufacturers.
We expect much of the additional complexity will come in the form of mapping rule sets against a company’s existing product catalog and associated prices, registration requirements, more deadlines, and continual changes to reporting methodology not to mention the reports themselves. The more requirements a manufacturer needs to meet, the higher the risk of something slipping through the cracks. In a perfect world, an overriding federal price reporting rule set would appear to be the best means to remove much of the complexity of dealing with what will likely become 50+ states and territories with disparate rule sets. However, as we all know so well, it is best to be careful what we wish for!
We recommend assigning the right individuals the task of managing drug price legislation responsibilities. Someone needs to own it. Have those responsible do the research. Make sure they carefully follow changes to requirements because they do occur frequently, then build and follow a solid SOP around the entire process. This has become a high-risk area for pharmaceutical manufacturers as many state regulatory agencies have assessed significant civil penalties for non-compliance.
If you fear your organization may be out of compliance, or if you’d like additional help to prepare for the ever-evolving landscape of state pharmaceutical price and financial disclosure laws, call us at 262-297-3007 or email your questions to info@prescriptionanalytics.com. We would be happy to schedule a free, no-obligation call to discuss the topic further and address any questions you may have.

Bob Devenport
Vice President of Government Pricing
Subscribe to receive our publications.
By signing up, you are agreeing with our privacy policy
