Continued Emergence of Drug Price Disclosure Legislation
Tips for staying compliant with an accelerating trend
States Have Gotten Aggressive with Fines Against Pharma Companies for Price Reporting Noncompliance
This paper, our third in a series that looks at the rising number of price transparency laws and their attention-grabbing compliance ramifications for pharma companies, offers tips on preparing for this new reality.
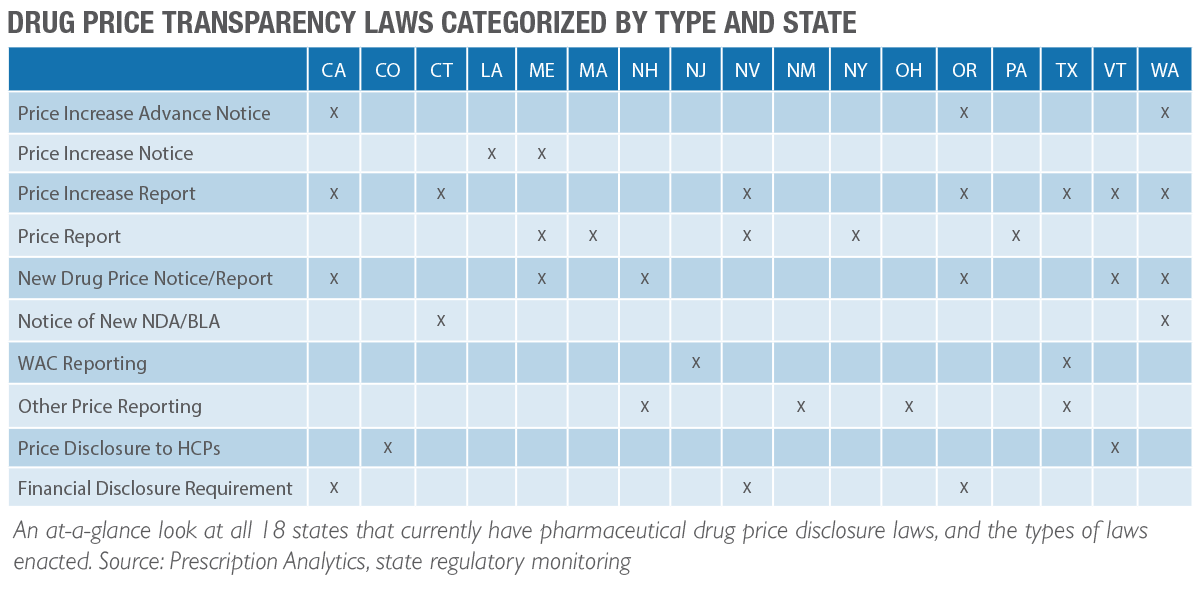
As of our last writing, there had been 14 states with enacted drug price transparency laws targeting pharmaceutical companies. As of this current writing, that number has increased to 18 states. Several reporting deadlines for these states have already passed. Any delinquent reports that have not been submitted in accordance with these deadlines are at risk of significant civil penalties. In fact, we are aware of states sending fine notices running into the millions of dollars. It is essential to know what needs to be reported, when to report it, and how to report it.
As we’ve mentioned in parts one and two of this series, there are steps you must take as a drug manufacturer to avoid noncompliance. Start by keeping an eye on what’s happening at the legislative level and consider taking the actions outlined in part two of this series. However, now that most of these laws and reporting requirements have gone into effect, it’s important to know when you need to act and be prepared to supply data ahead of the deadline for each state. As mentioned above, states are not being shy when it comes to penalizing noncompliant manufacturers with hefty fines.
Know Which State Reporting Requirements May Apply to You
As the number of states that have enacted price transparency laws increases so does the task of staying in compliance. Not only have the number of laws increased, but each of these states has its own set of nuances for each specific law that they have rolled out. The price increase you may need to report to one state, you may not need to report to another. It is important to read the fine print regarding each requirement for each state.
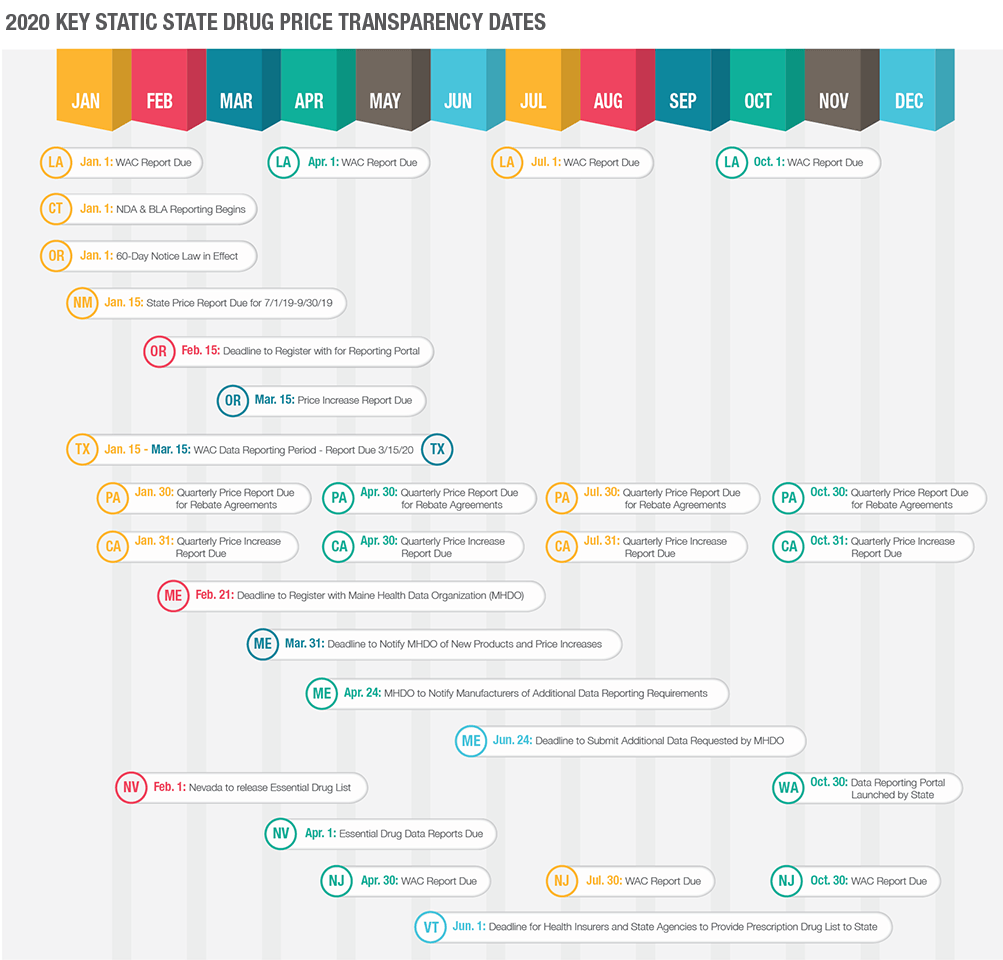
Recent and Approaching Deadlines
Most price transparency laws are in effect and state regulatory bodies are collecting data and verifying compliance among manufacturers. The first quarter of each year will likely be a busy time for manufacturers as many annual reporting deadlines fall within the first quarter. Given that we are well into the month of April, some of these reporting deadlines have already passed. If you have no knowledge of submitting reports to any states, odds are high that you may be out of compliance and potentially accruing a civil penalty as you read this. Below is a timeline illustrating various state reporting deadlines – note that this is only a portion of states and reporting deadlines.
California – Quarterly Reporting Deadlines
The state of California requires manufacturers to issue a 60-day advance notice of any WAC increases above the specified threshold. This advance notice is then required to be followed up with a price increase report in which the manufacturer must report certain data to the state via its online reporting portal. The deadline for the price increase reports falls on a quarterly basis. Any qualifying WAC increase must be reported by the end of the first full month following the quarter of the effective date of the WAC increase (e.g.: if the WAC increase is effective April 1, 2020, the report will be due July 31, 2020). The most recent deadline for any qualifying WAC increases from 4Q 2019 would have been due January 31, 2020. The next deadline for any qualifying WAC increases effective in 1Q 2020 will be due by April 30, 2020. If you, as a manufacturer, have any qualifying WAC increases from 2019 that have not been reported, you are out of compliance and may be accruing a penalty at the rate of $1,000 per day.
Texas – Annual Reporting Deadline
Texas is requiring manufacturers to report annually certain price data including WAC and AMP. Texas has already begun collecting the annual WAC reports from manufacturers. For 2020 reporting, the State set a deadline of March 15, 2020, to report all WAC data to the State. This means that if you have not reported WAC pricing to the state of Texas by now, you are out of compliance. In forthcoming years, this reporting deadline will likely be moved up to January of each year.
Texas will also have a WAC increase reporting requirement. This requirement has ongoing reporting deadlines. If a WAC increase meets certain thresholds as set forth by the State, the manufacturer will need to report certain data by no later than the 30th day following the effective date of the WAC increase.
Oregon – Annual Reporting Deadline
Oregon, like California, has several different aspects of price transparency legislation. Oregon requires a 60-day advance notice of qualifying WAC increases, WAC increase reporting, as well as new drug reporting for drugs that meet certain thresholds. Unlike California, Oregon requires all qualifying WAC increases to be reported on an annual basis for all WAC increases that took place during the prior calendar year. The most recent deadline was March 15, 2020, by which all qualifying WAC increases with an effective date during calendar year 2019 needed to be reported to the State. If you have any qualifying WAC increases form 2019 that have not been reported to Oregon, you are now out of compliance and potentially subject to a civil penalty.
Maine – Annual Reporting Deadline
All manufacturers selling prescription drugs into the state of Maine were required to register with the Maine Health Data Organization and create an account on the MHDO’s reporting portal by March 21, 2020. If your organization has not completed registration with the MHDO, you are out of compliance, and may be accruing a civil penalty. Once registered, your organization will have until March 31, 2020 to report if your organization had any qualifying WAC increases during the timeframe of September 19, 2019, through December 31, 2019. Once increases are reported, MHDO may reach out for additional information regarding the WAC increase. If so, the manufacturer will have until June 10, 2020, to report all requested information.
Nevada – Annual Reporting Deadline
The state of Nevada requires the annual submission of certain data regarding specific drugs that are considered essential in the treatment of asthma and diabetes. The Nevada Department of Health and Human Services is required to compile and release its “Essential Drug” list by February 1st of each year. Any manufacturer that has a drug on the Essential Drug list must provide a report regarding the drugs that appear on the list to the Nevada Department of Health and Human Services no later than April 1st of each year. If your organization has not reviewed this list yet, it is important to do so in order to determine if there are any reporting requirements that must have been met by April 1, 2020. Any out of compliance reporting is subject to a $5,000 per day administrative penalty.
Looking Forward
It is now more important than ever for manufacturers to begin actively managing the many price transparency requirements to which they may be subject. If your organization has not yet assigned an owner to these tasks and not yet begun the reporting process, there is a high probability that you may be out of compliance with one or more states and may be subject to significant fines and/or penalties. The price transparency requirements and deadlines that were outlined above are just a small sample and summary of the many laws faced by manufacturers. As was previously mentioned, states have already begun to impose severe fines for non-compliant manufacturers, sometimes $1,000 or even $5,000 per day per NDC.
If you fear your organization may be out of compliance, or if you’d like additional help on preparing for the changing environment of price disclosure laws and ensuring compliance, call us at 262-297-3007 or email your questions to info@prescriptionanalytics.com. We would be happy to schedule a free, no-obligation call to discuss the topic further and address any questions you may have.

Bob Devenport
Vice President of Government Pricing
Subscribe to receive our publications.
By signing up, you are agreeing with our privacy policy
