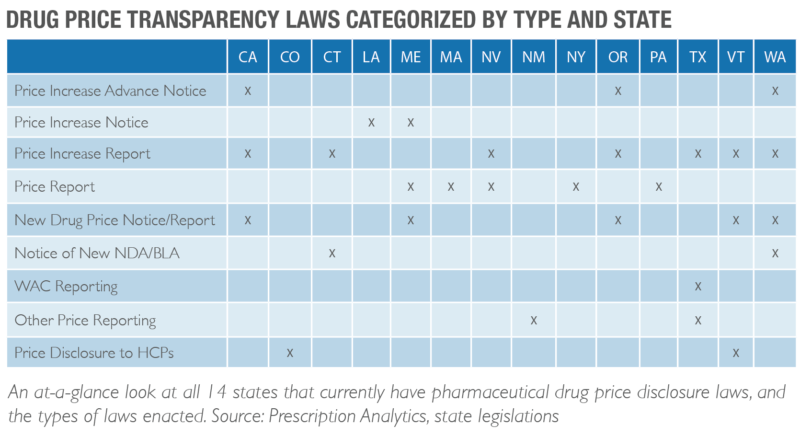
Continued Emergence of Drug Price Disclosure Legislation
Recommendations for Pharmaceutical Manufacturers
Define an owner. Opinions vary on who is responsible for ensuring compliance with transparency legislation requirements. Is it the Compliance department, Government Pricing, or some other department? Once you understand what price transparency compliance entails, you can more logically define who should own it. It may turn out to be more than one department. Wherever responsibility lands, formally designate one person to lead and take responsibility.
Create committees. Large pharma companies with subsidiaries may want to create multiple committees, each with a single owner, to manage compliance activities and report to a single leader who holds everyone accountable. Consider involving individuals from different departments or different branches of your company, ensuring diversity of knowledge and skills.
Hire well. Chances are that right now your organization does not have the people or resources in place to handle the coming increase in workload. Identify what it’s going to take, assuming you plan to manage reporting in-house. Determine if it’s necessary to outsource. If you do hire a third party, charge your people with being a knowledgeable, accessible resource to your vended partner.
Develop a consistent methodology. Just as companies have standard operating procedures in place for managing government pricing, so should they have an SOP for legislative compliance. New laws likely will require pharma companies to develop new reporting methodologies tailored to each state’s mandates.
Track and assess legislation. Keep a watchful eye on pending and new legislation, and know what may be required of you. Laws typically give manufacturers time to prepare, so take advantage of that time. Whether involving internal or external legal counsel, assign knowledgeable people. Counsel may need to interpret complex legislation with several moving parts.
Review strategies. New legislation may prompt you to reassess current and future pricing strategies. Know how legislation could affect drugs in your pipeline. You may decide it’s better to change pricing and avoid thresholds that trigger reporting.
Prepare for fallout. Legislation that mandates publication of pricing can lead to misunderstanding and swift backlash on social media, whether unfounded or not. Craft messages ahead of time. Corporate communications must be aware if their company is required to disclose its pricing and other corporate information. Be prepared for media inquiries and potential negative coverage.
If you’d like additional ideas on preparing for the changing environment of price disclosure laws, call us or email your questions to info@prescriptionanalytics.com. We uncover, manage and model data to help pharma companies gain confidence for making crucial strategic and operational business decisions.
3 AARP.org
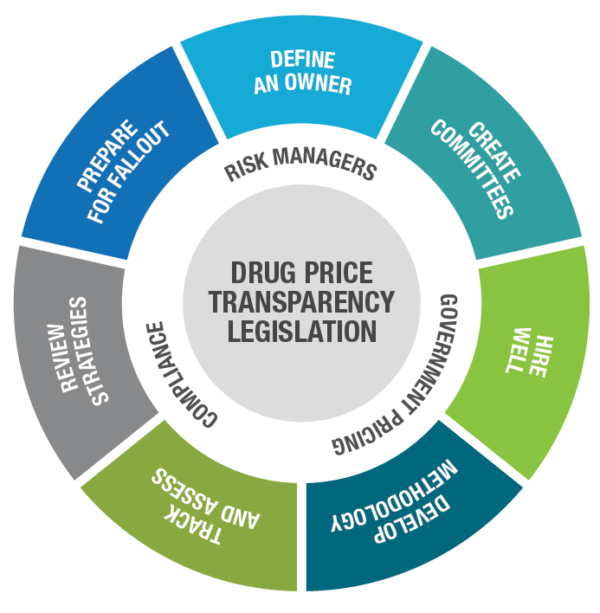
Pharma companies should assess each new transparency law and determine who will lead compliance and then take certain actions to mitigate financial and brand risk. Source: Prescription Analytics
Subscribe to receive our publications.
By signing up, you are agreeing with our privacy policy