Drug Price Disclosure Legislation
Four steps to staying ahead and staying compliant
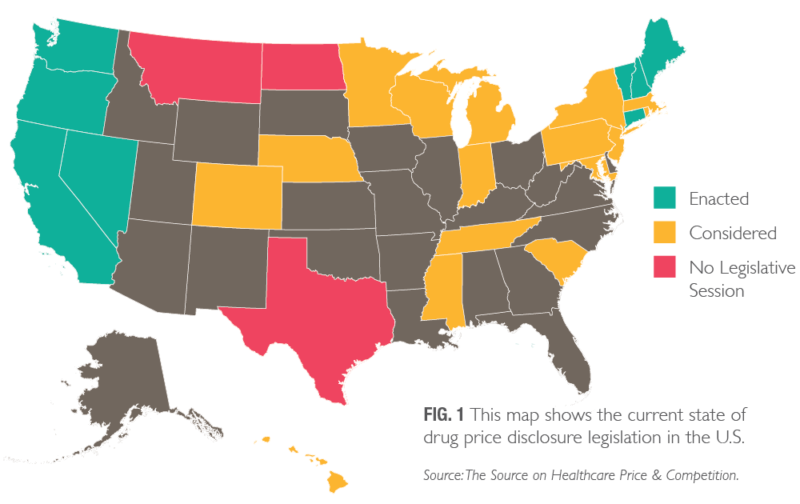
Many states have moved to enact mandatory pharmaceutical price disclosure laws that would increase price transparency to address prescription drug costs (Fig. 1, pg. 3). In 2018, states continued that trend, with 41 states having proposed plans to lower drug costs, including 23 considering transparency legislation.1, 2 Among the six states that passed legislation in 2018 were California and Oregon, notable not only for the pharmaceutical industry but also for the clients we serve.
The federal and state governments, holding differing views, have clashed over how to address prescription drug costs, leading legislators to propose hundreds of state laws aimed at lowering the cost of prescription drugs.
Price disclosure laws, while not directly targeted at lowering costs, will require transparency for certain price increases, which will reveal whether pricing has more to do with an assumption about a palatable price point than a pricing strategy based on science. These laws also may lead to increased pharmaceutical market competition.
In this paper, we will look at new laws in California and Oregon and what they require, as well as what manufacturers can and should do about this growing trend to remain compliant.
4 Ways to Prepare and Remain Compliant
- Ensure your compliance department is aware of all state laws requiring price and other information disclosures.
- Make sure that those tasked with price setting and/or changing are aware of price reporting disclosure triggers.
- Include as standard in your contract language that requires the customer to notify the company prior to a disclosure of information to a state regarding one of the company’s drugs, and also provide a copy of the actual disclosure.
- Corporate communications must be aware if the company is required to disclose its pricing and other corporate information and be prepared for media inquiries and potential negative coverage.
If you’d like additional ideas for how to prepare for the changing environment of price disclosure laws, call us or email your questions to info@prescriptionanalytics.com. We’re here to help you achieve your business objectives.
Subscribe to receive our publications.
By signing up, you are agreeing with our privacy policy